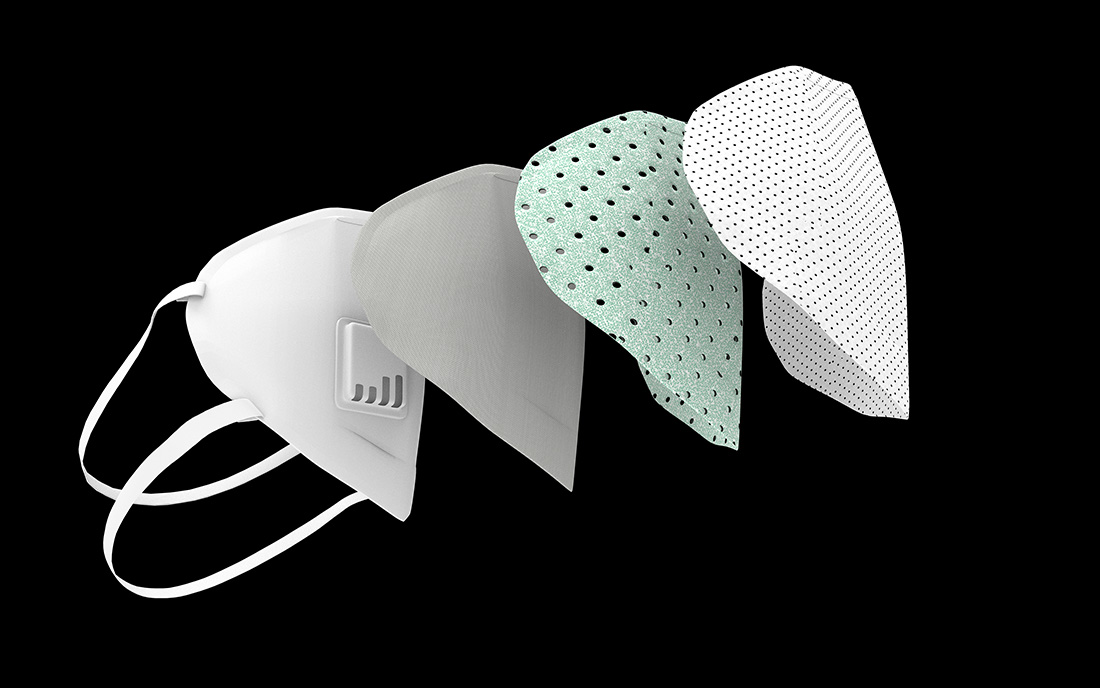
There are many different facemasks in the market today – from do-it-yourself cut and sew barrier masks to highly engineered products that require testing and certification to be used. In the age of COVID-19, the term “facemask” is, in some cases, being used generically, when there are different types of facemasks with varying degrees of protection.
Facemask types
- Non-medical facemask (minimum performance/utility facemask personal protection/procedure) – This option is a simple filter that covers the nose and mouth and provides protection against dust and pollen in the general public, home and workplace. These masks may be constructed of nonwovens, wovens and/or sponge materials.
- A purpose-built mask, which could include activated carbon for enhanced filtration efficiency, typically features pleats/folds to allow the user to expand the mask so it covers the face from the nose to under the chin. However, it can also be flat (not pleated).
Non-carbon pleated facemasks, which account for nearly 90 percent of disposable facial masks today, are rather loose and are not designed to protect the wearer against breathing in fine particulates or aerosolized contaminants. These masks are designed for relatively clean environments.
- FFP masks have similar specifications as a surgical mask, but are designed for industrial dust applications. The FFP masks come in three respirator ratings: FFP1 (80% efficiency for particles as small as 0.3 microns), FFP2 (94% efficiency) and FFP3 (99% efficiency). The FFP stands for “Filtering Face Piece.”
- Medical/surgical facemask – These masks are cleared for use in the U.S. by the Food and Drug Administration and have a significantly more complex testing protocol than the aforementioned mask types. In order to be called a medical/surgical mask, a minimum BFE (bacterial filtration efficiency) and PFE (particle filtration efficiency) rate is required.
Surgical masks are not designed or certified to prevent the inhalation of small airborne contaminants. They are, however, fluid resistant and provide the wearer protection against large droplets, splashes, or sprays of bodily or other hazardous fluids. These products protect the patient from the wearer’s respiratory emissions and are loose-fitting. They do not provide the wearer with a reliable level of protection from inhaling smaller airborne particles and are not considered respiratory protection. Leakage occurs around the edge of mask when the user inhales. These masks are designed for sterile environments and are disposable and should be discarded after each patient encounter.
- N95 respirator is a respiratory protective device designed to achieve a very close facial fit and is 95% efficient in the filtration of airborne particles. Note that the edges of the respirator are designed to form a seal around the nose and mouth and special training is needed to assure proper fitting.
Surgical N95 respirators are commonly used in healthcare settings and are a subset of N95 Filtering Facepiece Respirators (FFRs), often referred to as N95s. The N in N95 is a NIOSH (National Institute for Occupational of Safety & Health) classification indicating that the mask type is not for use where oil-based particles are present. Other classifications include R (oil resistant) and P (oil proof).
The similarities among surgical masks and surgical N95s are:
- They are tested for fluid resistance, filtration efficiency (particulate filtration efficiency and bacterial filtration efficiency), flammability and biocompatibility.
- They should not be shared or reused.
The nature and the type of face covers matter. In its most simple terms, a facemask protects others and a respirator protects the wearer.
Masks:
- Masks are loose fitting and cover the nose and mouth
- Masks are designed for one-way protection via capturing bodily fluid leaving the wearer
Respirators:
- Respirators are tight fitting masks and designed to create a facial seal
- Respirators are designed to protect the wearer
- Non-valved respirators provide good two-way protection by filtering both inflow and outflow
Facemask manufacturing
Both facemasks and respirators are manufactured using nonwoven fabrics made from materials like polyester, cellulose and polypropylene. They are also available in many different styles and grades depending on the level of protection the user requires.
The technology used in almost all masks for filtration is a meltblown nonwoven that is electrostatically charged. This inner nonwoven filter media uses a combination of mechanical filtration and electret charge to provide high efficiency due essentially to the charge and the mechanical tortuous path. The electrostatic charge is a critical feature of the mask in that it enhances the mechanical filtration efficiency from about 30-35% to over 95%.
Almost all meltblown fabrics are made from polypropylene and are rather fragile. They are often protected by layers of spunbonded nonwovens but can also be protected by a combination of needle punched and/or wetlaid nonwovens made of larger fibers. Most meltblown media for facemasks weigh about 20 to 30 g/m2, and the spunbond or other materials used in mask production are typically similar in weight.
The shear volume of masks needed for healthcare workers over the next months is likely to be over one billion masks (if used correctly as directed). The time required and volume capacity of the approved manufacturing methods for facemasks do not come close to meeting this demand. It would be like draining Lake Superior with a garden hose.
Reusability
I have doubts about the impact of reusing facemasks because there is no general cleaning of the mask; only a sterilization. What is to be made of body oils, spit and blood? These contaminants may be sterilized, but they are also blocking flow and adding resistance and potentially introducing other complications to the proper operation of the facemask.
As an example, the FDA has cleared Battelle’s technology to try to help meet mask demand during this time when supply is short. The Battelle CCDS (Critical Care Decontamination System) is designed to decontaminate N95 respirators using concentrated, vapor phase hydrogen peroxide. The respirators are exposed to vaporized hydrogen peroxide at the validated concentration level to decontaminate biological contaminants, including the SARS-CoV-2. The Battelle CCDS technology is said to be able to decontaminate the same respirator multiple times without degrading N95 respirator performance. However, this process is very labor intensive and time consuming and there is no final validation of the mask’s effectiveness after the process is complete.
The shear volume of masks needed for healthcare workers over the next months is likely to be over one billion masks (if used correctly as directed). The time required and volume capacity of the approved manufacturing methods for facemasks do not come close to meeting this demand. It would be like draining Lake Superior with a garden hose.
Given the “emergency use authorization” as part of the COVID-19 response here in the U.S., reuse may be the only option. However, after this crisis, the Battelle CCDS and other methods for reuse that are coming online need be revisited and questioned as to their completeness.
The future
There are many lessons to be learned from the COVID-19 outbreak. Filtration is a very important aspect of defense against this and future airborne viruses. Sourcing and availability certainly require added consideration and likely investment in new manufacturing assets. Technology specification is also an area worthy of discussion, as we consider the performance of different technologies and their appropriateness for different application environments.
A new barrier mask specification is in development currently with ASTM and NIOSH working on a test method for a new mask type that is validated as effective and protective in a “social distancing” scenario. This new mask specification aims to help minimize the potential for shortages of N95 mask types, which happened during COVID-19 due, in part, to these masks being purchased and used by the general public, leaving the healthcare community scrambling to acquire masks sufficient for healthcare applications.
Likewise, new indoor air quality standards linked to higher efficiency filtration media may be on the horizon for institutional buildings to protect tenants. New technologies for filtration are also being introduced daily to combat viruses, including new resins, nonwoven technologies and new equipment to manufacture and test.
Medical groups and the government are leaning very hard on filtration at this moment in time, and I believe the filtration community is ready to step up to the challenge.
As INDA’s director of education and technical affairs, Chris presents regular training related to nonwovens and filter media from INDA headquarters in Cary, NC. For more information about upcoming training opportunities, visit inda.org/education.
* International Filtration News is owned by INDA, Association of the Nonwoven Fabrics Industry.