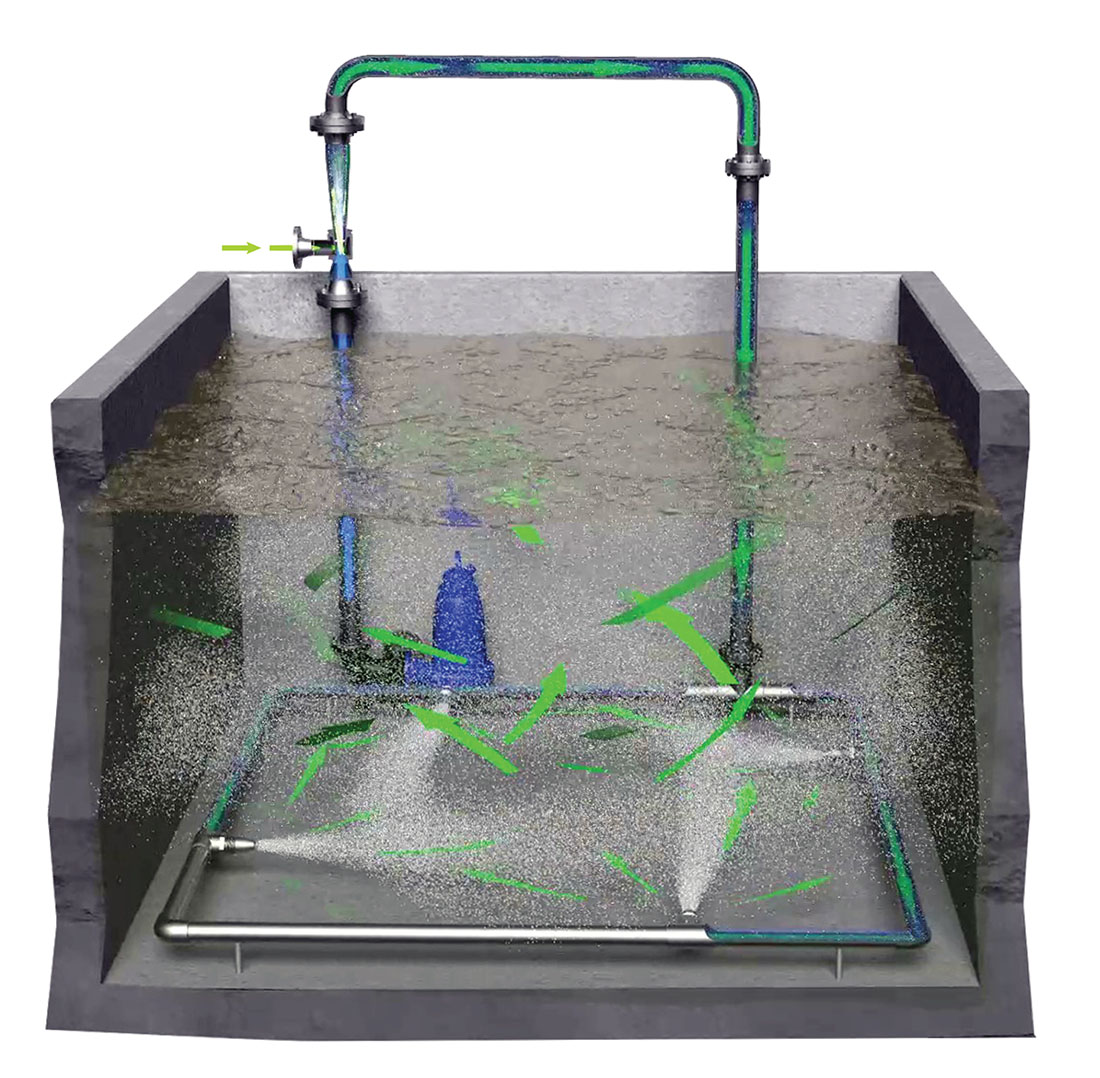
Biologically active filtration (BAF) is getting a substantial boost in many water reuse and wastewater treatment applications from the oxidation power of ozone. Ozone has a higher oxidation potential than chlorine, hydrogen peroxide, hypochlorous acid, and any other disinfectant on the market except fluorine. The use of ozone has increased dramatically in recent years, and appreciation is growing not only for its disinfection and sanitation potential, but also for its ability to augment filtration systems, especially BAF.
The multi-barrier approach is always a good idea. With ozone, it’s a great idea.
Powerful oxidant
Ozone is created by breaking apart oxygen molecules (O2) with a jolt of electrical energy and recombining their atoms into O3. The three-atom molecule is unstable, quick to release its extra atom to other molecules that often break their own internal bonds to link to the free oxygen. The rapid oxidation caused by ozone blasts open bacteria and viruses, rips apart long-chain molecules like pharmaceuticals and pesticides, and binds up ions like iron and manganese. Then, in moments, the ozone has fully reacted and is gone.
Oxidizing iron or manganese with ozone allows the metals to flocculate out of solution to be captured in settling tanks or filters. Taste and odor compounds, as well as poisonous chemicals like cyanotoxins, are neutralized and destroyed. The stage is also set for highly efficient, and highly effective, advanced oxidation processes (AOPs) like ozone plus UV or ozone plus hydrogen peroxide.
But perhaps the most exciting combination of oxidation and filtration is the use of ozone before biologically active filtration. On a microscopic level, ozone breaks long-chain molecules into “bite-size” pieces, digestible by the active microbes on a BAF substrate.
A concern with ozone is the formation of bromate in the presence of bromine. However, the brief residence time needed in SSI systems minimizes the formation of bromate.
More ozone
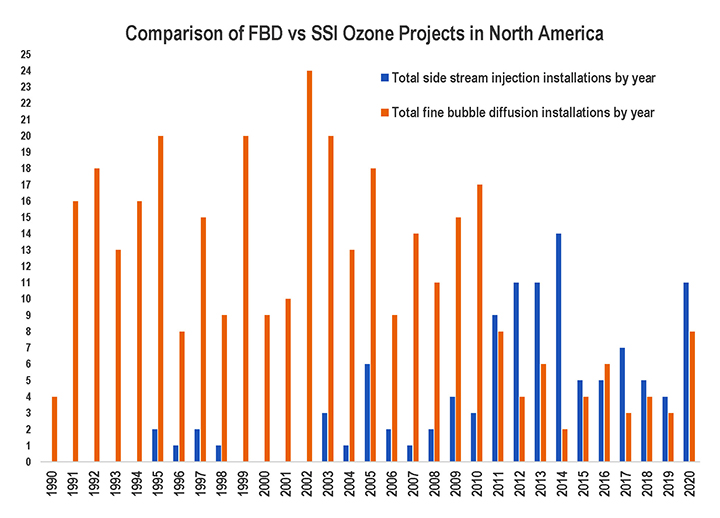
For generations, ozone was created by exposing treated air—dried, filtered, and cooled—to a high electrical charge. In the 1990s, a new generation of ozone generators was developed. The high-efficiency systems are fed by liquid oxygen (LOx), eliminating the need for pretreating the air and boosting output to ozone concentrations of 10, 12, even approaching 20%.
High-concentration ozone streams allowed many engineers to leave behind fine bubble diffusers (FBD), which break ozone streams into small bubbles released at depths of 21 feet to optimize conditions for them to dissolve into solution. Instead, sidestream injection (SSI) systems stepped into the spotlight, diverting a small percentage of the total flow through a venturi injector. Inside the venturi, the stream is compressed, then allowed to expand again. Thanks to the Venturi effect, the changes in rate and pressure create a vacuum that draws ozone into the flow and mixes it into solution.

The ozonated sidestream is then returned to the main flow through a pipeline flash reactor (PFR), which actively mixes the concentrated solution into the rest of the water in a matter of a few feet of pipelined mixing features, or through a basin nozzle manifold, which jets the ozonated sidestream into a shallow mixing basin with specially designed and positioned nozzles that optimize mixing.
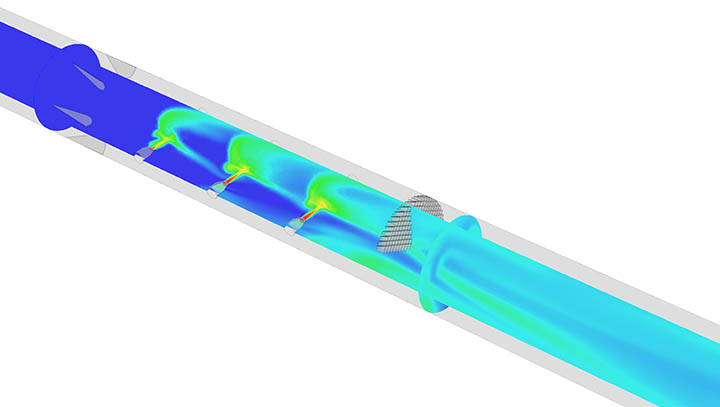
Ozone injection mixing systems are among the key drivers of computational fluid dynamics (CFD) research and application. CFD analysis guides our engineers in sizing, positioning and orienting mass transfer nozzles. The technology was also critical in developing the PFR+, which integrates curved vanes and a special mixing grid to enhance PFR performance and eliminate the need for a static mixer in ozone systems.
Both PFR and basin nozzle manifold systems can achieve mass transfer rates of 95% or more. That is important for several reasons. First, you want as much as possible of the ozone you generated to control contaminants. Second, free ozone coming into contact with the bacteria on the BAF substrate could damage the beneficial microbial community, so it is important to use up the ozone in disinfection—yielding dissolved oxygen in the water, which enhances bacterial growth—or remove the undissolved ozone with a degas system.
Enhancing downstream systems
Ozone’s chain-breaking oxidation allows BAF to work at its highest potential, and because it prepares complex contaminants for digestion, it also helps minimize contaminated backflush brines that require disposal. Organisms or dead cells that can create biological oxygen demand (BOD) are similarly neutralized, broken, and prepared for digestion.

Breaking up organic molecules also helps increase light transmittance, which enhances the efficacy of UV AOP systems downstream.
A concern with ozone is the formation of bromate in the presence of bromine. However, the brief residence time needed in SSI systems minimizes the formation of bromate. Ozone can be the heart of a precision system, managed with automated sensors that can detect its presence and adjust system settings to optimize sidestream flow, ozone rate, and contact time to meet specs for specific contaminants and levels of contamination. And SSI systems can handle greater turndown than FBD systems can, either by bringing skids on- or off-line, or by simply reducing flow as needed, confident that injection and mixing will continue to function as needed.
In all, ozone can be an ideal partner for biologically active filtration as well as a wide range of downstream filtration technologies. More information on ozonation for water quality applications can be found at bit.ly/Ozonation.